DENTAL SCANNER DESIGN
A startup medical company focusing on continuous cardiac monitoring needed design, development and engineering of a wearable/external arrhythmia monitoring device.
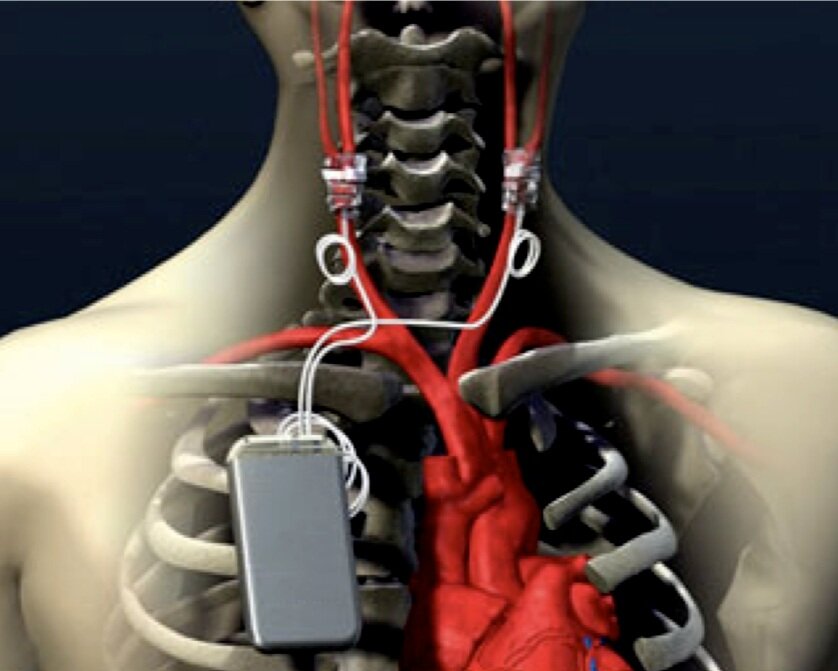
CARDIAC MONITOR
A startup medical company focusing on continuous cardiac monitoring needed design, development and engineering of a wearable/external arrhythmia monitoring device.
PAIN MANAGEMENT DEVICE
Lucid performed early-stage development work on Nevro’s first chronic pain device.
INSULIN PUMP
An insulin pump maker was expanding their systems beyond their core business of external insulin pumps.
NEUROSTIMULATION SYSTEM
A neurostimulation company focused on developing therapeutic systems for obesity, hypertension and diabetes, asked Lucid for product development expertise…
NEUROMODULATION DEVICE
A well-funded implantable technology development company focusing on solutions for hypertension and heart failure needed development of a proof of concept for a delivery tool for deployment of active implantable lead.
ATHERECTOMY DEVICE
An atherectomy device manufacturer had a controller board being manufactured in Europe with a modified firmware code base…
DENTAL PROCESS DESIGN
A startup medical company focusing on continuous cardiac monitoring needed design, development and engineering of a wearable/external arrhythmia monitoring device.
CARDIAC RHYTHM MANAGEMENT (CRM) DEVICE
A leading Fortune 500 medical device technology company needed to develop functional prototypes for internal and external neuromodulation devices.